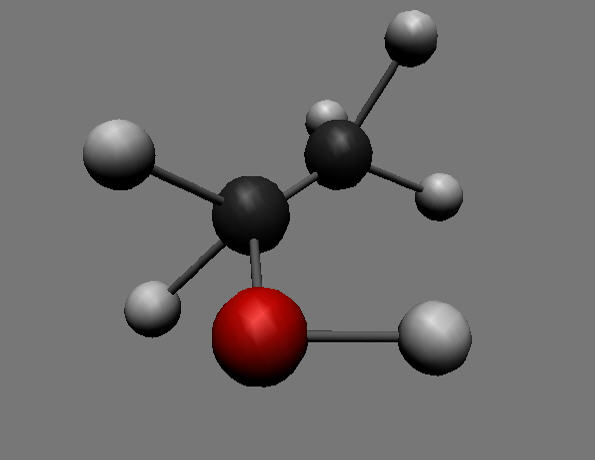
Ethanol molecule
Ethanol molecule
TLF ID R6972
This is a colour image of a model of a molecule of ethanol, CH₃CH₂OH. In this model, atoms are represented by coloured spheres held together by grey rods that represent covalent bonds. The molecule contains two carbon atoms (the black spheres), one oxygen atom (the red sphere) and six hydrogen atoms (the grey-white spheres).